A-403, Mathuria Apts, Sir M.V.Road, Andheri (East), Mumbai - 400069.
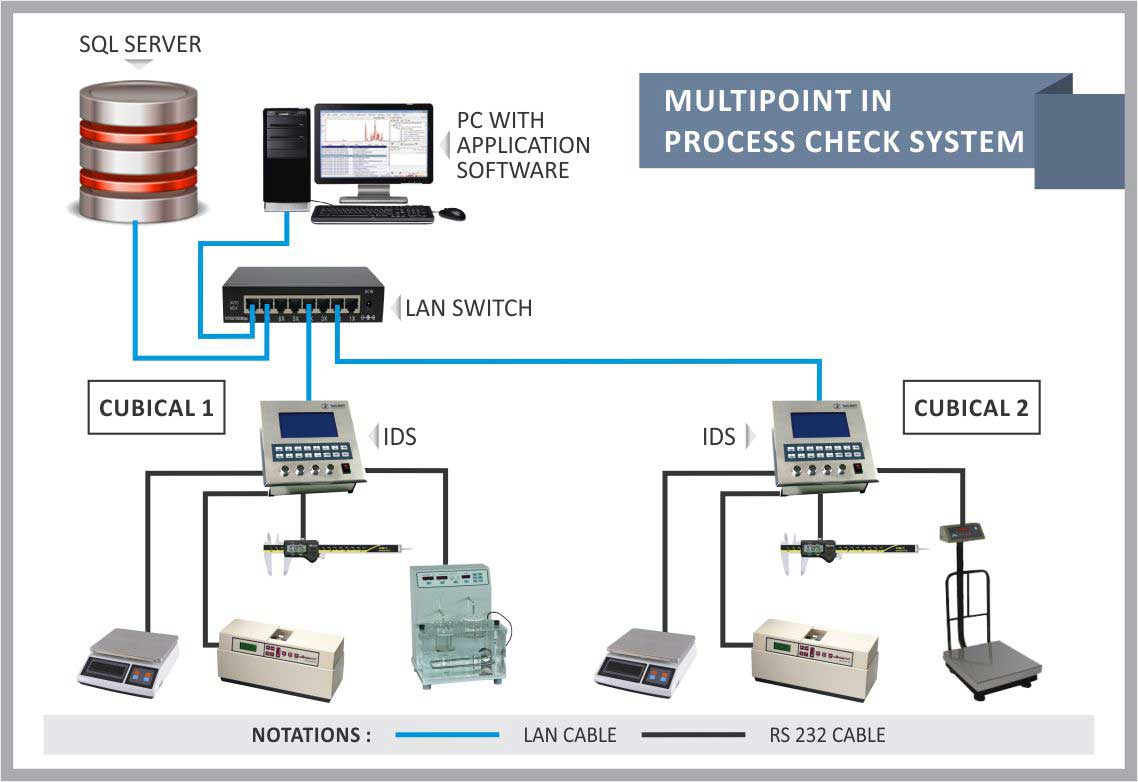
This system is designed for tablet/capsule production area of pharmaceutical companies.
Individual weight variation tests, group weight variation tests, thickness variation tests, dimension variation tests, dissolution tests and other statistical quality control tests are an important part of IPQC (In Process Quality Control) checks.
The equipment such as weighing scales, hardness tester, dissolution tester, vernier caliper, temperature sensor, humidity sensor etc. are connected to the hardware (TOUCHSCREEN) in various areas such as IPQC, Production, Packaging etc.
The collected data is stored in centralized database and MIS report can be generated as per users’ requirement.
To maintain accuracy of the balance, the electronic data management system has the facility to perform weighing balance calibration as and when required.
Since IPQC is a critical area, the electronic data transfer can help on considerably saving the time and efforts required for testing.
The system supports 21 CFR part 11 compliance.

A-403, Mathuria Apts, Sir M.V.Road, Andheri (East), Mumbai - 400069.