Incrency – In-Process Check System
This system is designed for tablet/capsule production areas of pharmaceutical companies. It facilitates various tests crucial for In Process Quality Control (IPQC) checks, including individual weight variation tests, group weight variation tests, thickness variation tests, dimension variation tests, dissolution tests, and other statistical quality control tests. Equipment such as weighing scales, hardness testers, dissolution testers, vernier calipers, temperature sensors, humidity sensors, etc., are connected to the hardware (TOUCHSCREEN) in areas such as IPQC, Production, and Packaging. The system collects data from these devices and stores it in a centralized database, allowing for MIS reports to be generated as per user requirements. To maintain the accuracy of the balance, the system has the facility to perform weighing balance calibration as and when required. Given the critical nature of IPQC, electronic data transfer can significantly save time and effort required for testing.



Salient Features

Validation in the Sytem Eliminates Errors & Data Inconsistencies Completely

Safe And Permanent Data Storage Faciliates Completely Paperless Operations
Web Based System enables the option to be used on local server or over the internet

Customised Reports ensure you get the reports as per your requirement

System Architecture
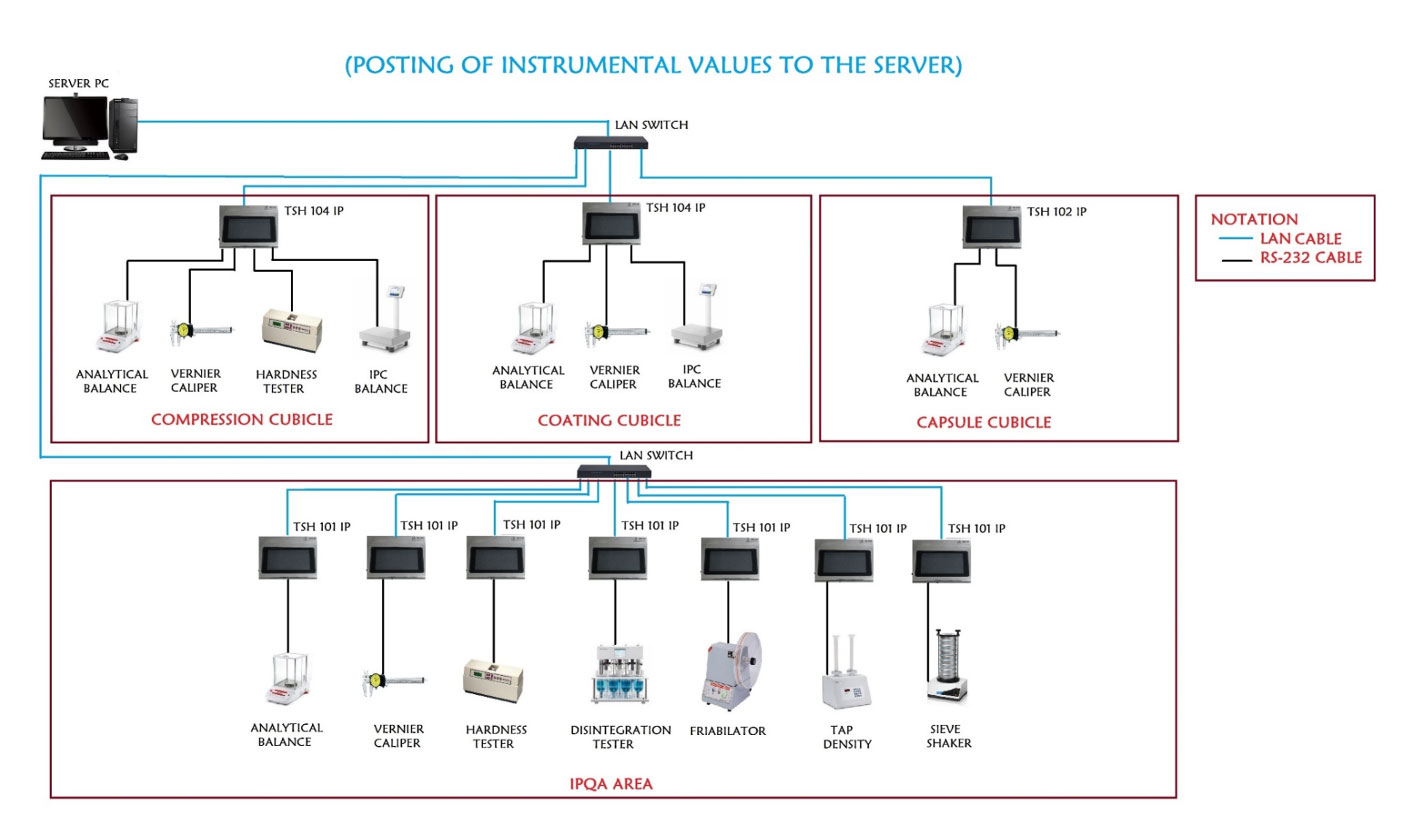
Application Software Features
-
Data collection from multiple equipments placed in operation area through electronic hardware over LAN network.
-
Various master e.g. User, Product, Std wt box etc.
-
Report generation as per user format.
-
Authorized print and reprint.
-
Various securities as stipulated in US FDA 21 CFR part 11 compliance.
Electronic Hardware Specfications
- 5 Inch Touch Display
- Touch Type: 5 Finger Capacitive Touch.
- Processor: Quad Core 1.2GHz 64bit CPU ARM Cortex A53 (ARMv8) cluster.
- RAM: 1 GB LPDDR2 (900 MHz)
- Ethernet: 10/100 Ethernet
- Screen Resolution: 800 x 480 RGB LCD Display
- Operating temperature: -20 to +70 degrees centigrade
System Trusted By

Halol

Goa & Indore

Indore

Indore

Sikkim

Plant Locations:
Hosur, Veersandra, Goa, Sikkim & Pondicherry

Plant Locations:
Halol, Dadra & Nagar Haveli & Sikkim

Plant Locations:
Baddi, Goa, Indore, Sikkim & Patalganga

Dahej & Aurangabad

Bangalore
Frequently Asked Questions
What types of tests can be performed using the In-Process Check System in the tablet/capsule production area?
The In-Process Check System is equipped to perform a variety of quality control tests including individual weight variation tests, group weight variation tests, thickness variation tests, dimension variation tests, and dissolution tests. These tests are crucial for maintaining the quality of tablets and capsules during production.
How does the In-Process Check System handle data management and reporting?
Data collected from the various tests and equipment is stored in a centralized database. This allows for efficient management and retrieval of data, enabling users to generate Management Information System (MIS) reports based on specific requirements.
What equipment is integrated with the In-Process Check System for IPQC checks?
The system utilizes a range of equipment such as weighing scales, hardness testers, dissolution testers, vernier calipers, temperature sensors, and humidity sensors. These are connected to the system via a touchscreen interface that facilitates ease of use across various departments like IPQC, production, and packaging.
How does the system ensure the accuracy of weighing balances and what benefits does electronic data transfer provide?
To maintain high accuracy, the system includes a facility for calibrating weighing balances as needed. Additionally, the use of electronic data transfer within the IPQC process significantly reduces the time and effort required for conducting tests, thereby enhancing overall efficiency and reliability in quality control.
Phone
(+91) 7506029981
Address
A-403, Mathuria Apts, Sir M.V.Road, Andheri (East), Mumbai - 400069.
