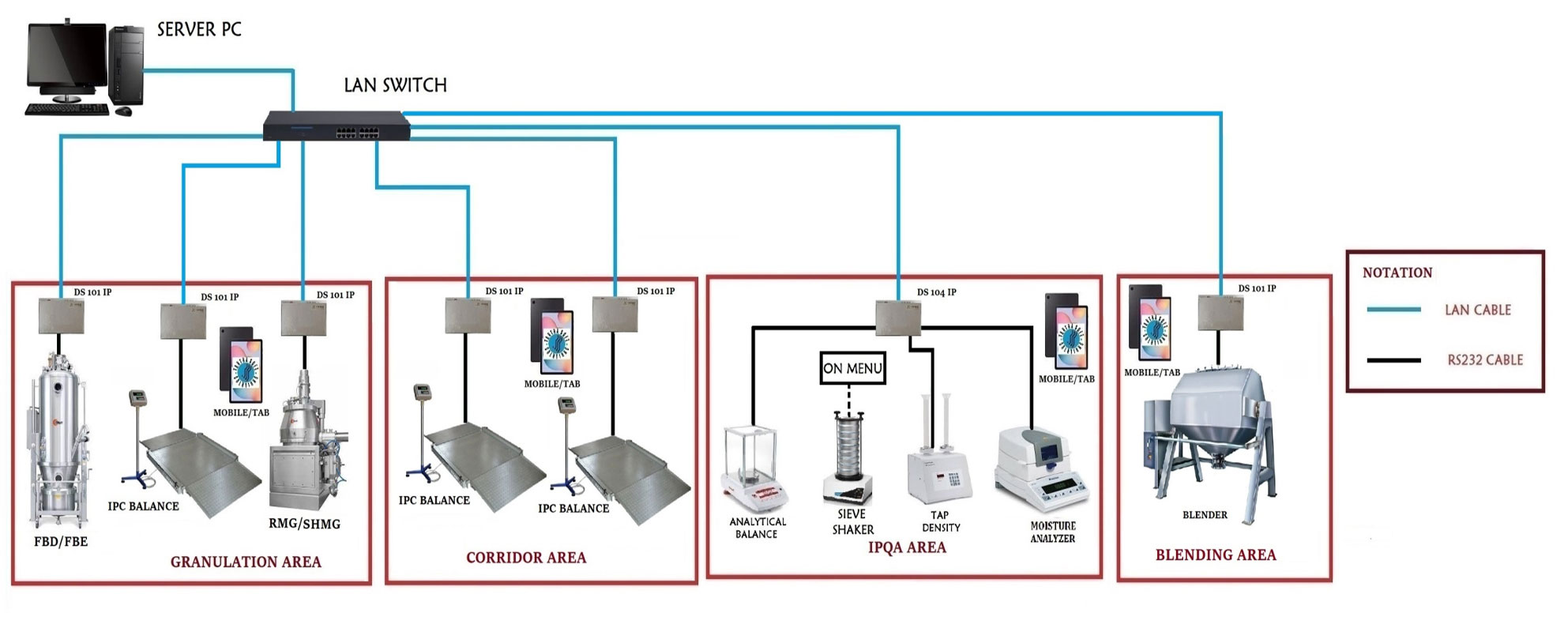
Incrency – Granulation System
This system is a pivotal solution designed to optimize both dry and wet granulation processes in tablet and capsule manufacturing. By closely monitoring Critical Process Parameters (CPP) and Critical Quality Attributes (CQA), the system ensures precise management of granulation. It also vigilantly tracks the environmental conditions within the manufacturing areas, preventing process failures related to environmental factors. The system supports efficient management of raw and bulk materials by adhering to the First-In-First-Out (FIFO) principle. These integrated features facilitate the generation of Right First Time (RFT) reports, which not only reduce the review period but also contribute to the delivery of high-quality products.



Salient Features

Validation in the Sytem Eliminates Errors & Data Inconsistencies Completely

Safe And Permanent Data Storage Faciliates Completely Paperless Operations
Web Based System enables the option to be used on local server or over the internet

Customised Reports ensure you get the reports as per your requirement

Application Software Features
- Recipe master.
- Area/room master has instruments and environmental parameters (Temperature, RH and Differential Pressure).
- Approval of product required. Only approved product’s batch can be started. This avoids inactive products from execution.
- Verification of availability of instruments required for the product during batch setting.
- Batch Release/Reject by QA after Yield Reconciliation.
- Status of every area can be viewed.
- FIFO execution for Lots.
Hardware Features (Tab)
-
QR code verification for every room assures accuracy of data.
-
Area and instrument clearance before starting batch.
-
QA verification after every line clearance.
-
Values entered/captured from instrument cannot be edited.
-
Restriction from proceeding if values (instrumental / environmental) are out of pre-set tolerances.
-
Exception log to file for every out of limit value.
-
Server date and time captured gives contemporaneous data.
-
User can access Stage specific General Instructions. IPC selection and IPC label generation for each stage
-
Yield Reconciliation.
System Trusted By

Halol

Goa & Indore

Indore

Indore

Sikkim

Plant Locations:
Hosur, Veersandra, Goa, Sikkim & Pondicherry

Plant Locations:
Halol, Dadra & Nagar Haveli & Sikkim

Plant Locations:
Baddi, Goa, Indore, Sikkim & Patalganga

Dahej & Aurangabad

Bangalore
Frequently Asked Questions
What is the E-Granulation System and what processes does it support?
The E-Granulation System is a sophisticated technology designed to enhance the granulation process in pharmaceutical manufacturing, specifically for tablet and capsule production. It supports both dry and wet granulation methods, ensuring efficient and high-quality production.
Can the E-Granulation System help in managing environmental factors in production areas?
Yes, the E-Granulation System plays a crucial role in monitoring environmental conditions such as temperature and humidity within the manufacturing areas. This capability helps prevent process failures that could be caused by unfavorable environmental conditions, thus maintaining the stability and quality of the production process.
How does the E-Granulation System ensure quality in granulation processes?
The system ensures quality by meticulously monitoring Critical Process Parameters (CPP) and Critical Quality Attributes (CQA). This allows for precise control over the granulation process, adhering to stringent quality standards and ensuring the consistency and integrity of the final product.
What are the benefits of using the E-Granulation System in terms of material handling and production efficiency?
The E-Granulation System enhances material handling efficiency by implementing the First-In-First-Out (FIFO) principle, which helps in controlling the movement of raw and bulk materials effectively. This system also facilitates the generation of Right First Time (RFT) reports, reducing the time needed for review and ensuring the timely delivery of high-quality pharmaceutical products.
Phone
(+91) 7506029981
Address
A-403, Mathuria Apts, Sir M.V.Road, Andheri (East), Mumbai - 400069.